within a group what happens to the atomic radius
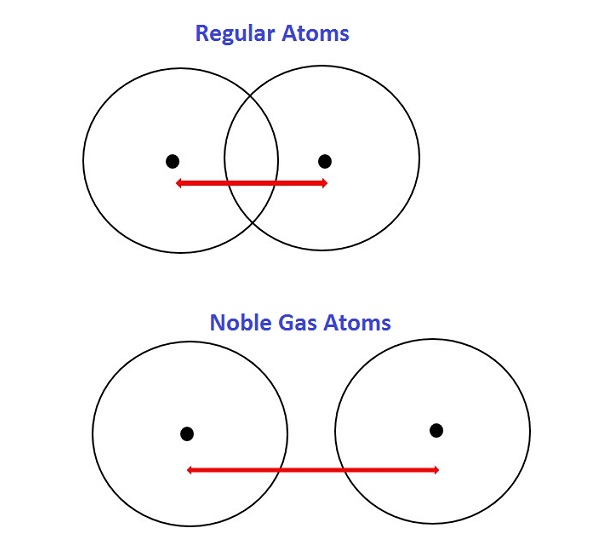
Need information on diminutive radius trends? What's the trend for atomic radius? In this guide, nosotros'll clearly explain atomic radius trends and how they work. We'll also discuss exceptions to the trends and how you can apply this information as part of a broader agreement of chemistry. Before we dive into diminutive radius trends, let'southward review some bones terms. An atom is a bones unit of measurement of a chemical element, such as hydrogen, helium, potassium, etc. A radius is the distance between the middle of an object and its outer edge. An atomic radius is one-half the altitude between the nuclei of two atoms. Atomic radii are measured in picometers (one picometer is equal to one trillionth of a meter). Hydrogen (H) has the smallest average atomic radius at well-nigh 25 pm, while caesium (Cs) has the largest boilerplate radius at about 260 pm. There are ii main atomic radius trends. I diminutive radius trend occurs as yous move left to right across the periodic tabular array (moving inside a period), and the other trend occurs when you motility from the top of the periodic table down (moving within a group). Below is a periodic table with arrows showing how atomic radii modify to help you understand and visualize each atomic radius trend. At the terminate of this section is a chart with the estimated empirical atomic radius for each chemical element. The offset diminutive radius periodic trend is that atomic size decreases as yous motility left to correct across a menstruation. Within a period of elements, each new electron is added to the same shell. When an electron is added, a new proton is too added to the nucleus, which gives the nucleus a stronger positive charge and a greater nuclear allure. This ways that, every bit more protons are added, the nucleus gets a stronger positive charge which and so attracts the electrons more strongly and pulls them closer to the atom's nucleus. The electrons being pulled closer to the nucleus makes the cantlet'south radius smaller. Comparing carbon (C) with an diminutive number of 6 and fluorine (F) with an atomic number of 9, we can tell that, based on atomic radius trends, a carbon cantlet will take a larger radius than a fluorine cantlet since the three additional protons the fluorine has volition pull its electrons closer to the nucleus and shrink the fluorine's radius. And this is true; carbon has an average diminutive radius of most 70 pm while fluorine's is about l pm. The 2d atomic radius periodic trend is that atomic radii increase equally you lot motion downwards in a group in the periodic tabular array. For each group you motion downwards, the atom gets an additional electron shell. Each new shell is further away from the nucleus of the cantlet, which increases the diminutive radius. While y'all may think the valence electrons (those in the outermost shell) would be attracted to the nucleus, electron shielding prevents that from happening. Electron shielding refers to a decreased attraction between outer electrons and the nucleus of an cantlet whenever the atom has more than ane electron shell. And then, because of electron shielding, the valence electrons don't get particularly close to the center of the atom, and because they tin't get that close, the atom has a larger radius. Every bit an example, potassium (Chiliad) has a larger average atomic radius (220 pm)than sodium (Na) does (180 pm). The potassium atom has an extra electron shell compared to the sodium cantlet, which means its valence electrons are farther from the nucleus, giving potassium a larger atomic radius. The two atomic radius trends nosotros discussed above are true for the majority of the periodic table of elements. Notwithstanding, there are a few exceptions to these trends. 1 exception is the noble gases. The vi noble gases, in grouping 18 of the periodic table, are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The noble gases are an exception because they bond differently than other atoms, and noble gas atoms don't get equally close to each other when they bond. Because atomic radius is one-half the altitude between the nuclei of two atoms, how close those atoms are to each other affects atomic radius. Each of the noble gases has their outermost electron shell completely filled, which means multiple noble gas atoms are held together past Van der Waals forces rather than through bonds. Van der Waals forces aren't as stiff every bit covalent bonds, then 2 atoms continued by Van der Waals forces don't get as close to each other every bit two atoms continued past a covalent bond. This means the radii of the noble gases would be overestimated if we attempted to find their empirical radii, and so none of the noble gases have an empirical radius and thus don't follow the atomic radius trends. Below is a very simplified diagram of four atoms, all nearly the same size. The top 2 atoms are connected past a covalent bond, which causes some overlap between the atoms. The bottom two atoms are noble gas atoms, and they are connected by Van der Waals forces that don't allow the atoms to get as close together. The red arrows stand for the distance between the nuclei. One-half of this altitude is equal to atomic radius. As you tin meet, even though all four atoms are about the same size, the element of group 0 radius is much larger than the radius of the other atoms. Comparing the two radii would brand the noble gas atoms wait bigger, even though they're not. Including noble gas radii would give people an inaccurate idea of how big noble gas atoms are. Because noble gas atoms bond differently, their radii can't be compared to the radii of other atoms, then they don't follow atomic radius trends. Other exceptions include the lanthanide series and actinide serial at the bottom of the periodic tabular array. These groups of elements differ from much of the rest of the periodic tabular array and don't follow many trends the other elements do. Neither series has a clear diminutive radius trend. While y'all probably won't demand to know the diminutive radius of various elements in your solar day-to-day life, this information can yet be helpful if yous're studying chemistry or another related field. Once you understand each key diminutive radius period trend, it makes it easier to empathize other information about the elements. For example, y'all tin call up that noble gases are an exception to the diminutive radius trends because they have a full outer electron trounce. These outer electron shells as well make the noble gases inert and stable. That stability can be handy. For example, balloons are typically filled with helium, not hydrogen, because helium is much more stable and therefore less flammable and safer to use. You lot can also utilize diminutive radii to gauge how reactive different elements will be. Atoms with smaller radii are more reactive than atoms with larger radii. The halogens (in group 17) accept the smallest average radii in the periodic table. Fluorine has the smallest atomic radius of the halogens (which makes sense based on the trends), and that makes it highly reactive. Just adding fluorine to water will produce flames as the fluorine turns into a gas. In that location are two main diminutive radius trends. The offset atomic radius periodic trend is that atomic radii increment every bit y'all move down in a group. This is due to electron shielding. When an additional shell is added, those new electrons are farther from the atom's nucleus, which increases atomic radius. The second diminutive radius periodic trend is that diminutive size decreases moving left to right across a period considering the atom's stronger positive charge due to having more protons attracts the electrons more strongly and pulls them closer to the nucleus, reducing the size of the atom. There are a few exceptions to these trends, noticeably the noble gases which don't form bonds the fashion most other atoms do, and the lanthanide and actinide series. Yous tin can utilize this information to better understand the periodic table, how atoms bond, and why certain elements are more reactive than others. Demand to brush upwardly on your molecular chemical science? Review the different kinds of hydrates, how electronegativity works, and the uses (and limitations) of the Bohr Atomic Model. Taking advanced chemistry and need some assist? We take study guides for AP Chem and IB Chemistry, as well as a full general Regents Chemistry review for New York loftier school students. Dipping your toe into the wonderful world of biochemistry? Learn well-nigh the half-dozen types of enzymes and the chemical limerick of nucleotides.
What Are the Diminutive Radius Trends? What Causes Them?

Atomic Radius Tendency ane: Atomic Radii Subtract From Left to Right Across a Menses
Atomic Radius Tendency ii: Atomic Radii Increase equally Y'all Motility Down a Group
Empirical Diminutive Radii
Diminutive Number Symbol Element Proper noun Empirical Atomic Radius (pm) 1 H Hydrogen 25 2 He Helium No data 3 Li Lithium 145 4 Be Beryllium 105 5 B Boron 85 6 C Carbon lxx 7 N Nitrogen 65 8 O Oxygen 60 9 F Fluorine fifty ten Ne Neon No data 11 Na Sodium 180 12 Mg Magnesium 150 13 Al Aluminum 125 fourteen Si Silicon 110 15 P Phosphorus 100 sixteen South Sulfur 100 17 Cl Chlorine 100 18 Ar Argon No data xix K Potassium 220 xx Ca Calcium 180 21 Sc Scandium 160 22 Ti Titanium 140 23 V Vanadium 135 24 Cr Chromium 140 25 Mn Manganese 140 26 Fe Iron 140 27 Co Cobalt 135 28 Ni Nickel 135 29 Cu Copper 135 30 Zn Zinc 135 31 Ga Gallium 130 32 Ge Germanium 125 33 Every bit Arsenic 115 34 Se Selenium 115 35 Br Bromine 115 36 Kr Krypton No information 37 Rb Rubidium 235 38 Sr Strontium 200 39 Y Yttrium 180 40 Zr Zirconium 155 41 Nb Niobium 145 42 Mo Molybdenum 145 43 Tc Technetium 135 44 Ru Ruthenium 130 45 Rh Rhodium 135 46 Pd Palladium 140 47 Ag Argent 160 48 Cd Cadmium 155 49 In Indium 155 50 Sn Tin 145 51 Sb Antimony 145 52 Te Tellurium 140 53 I Iodine 140 54 Xe Xenon No information 55 Cs Caesium 260 56 Ba Barium 215 57 La Lanthanum 195 58 Ce Cerium 185 59 Pr Praseodymium 185 lx Nd Neodymium 185 61 Pm Promethium 185 62 Sm Samarium 185 63 European union Europium 185 64 Gd Gadolinium 180 65 Tb Terbium 175 66 Dy Dysprosium 175 67 Ho Holmium 175 68 Er Erbium 175 69 Tm Thulium 175 70 Yb Ytterbium 175 71 Lu Lutetium 175 72 Hf Hafnium 155 73 Ta Tantalum 145 74 W Tungsten 135 75 Re Rhenium 135 76 Os Osmium 130 77 Ir Iridium 135 78 Pt Platinum 135 79 Au Golden 135 80 Hg Mercury 150 81 Tl Thallium 190 82 Pb Atomic number 82 180 83 Bi Bismuth 160 84 Po Polonium 190 85 At Astatine No data 86 Rn Radon No data 87 Fr Francium No data 88 Ra Radium 215 89 Air conditioning Actinium 195 xc Th Thorium 180 91 Pa Protactinium 180 92 U Uranium 175 93 Np Neptunium 175 94 Pu Plutonium 175 95 Am Americium 175 96 Cm Curium No data 97 Bk Berkelium No data 98 Cf Californium No information 99 Es Einsteinium No information 100 Fm Fermium No data 101 Doc Mendelevium No data 102 No Nobelium No information 103 Lr Lawrencium No data 104 Rf Rutherfordium No data 105 Db Dubnium No data 106 Sg Seaborgium No data 107 Bh Bohrium No data 108 Hs Hassium No data 109 Mt Meitnerium No data 110 Ds Darmstadtium No information 111 Rg Roentgenium No information 112 Cn Copernicium No information 113 Nh Nihonium No data 114 Fl Flerovium No data 115 Mc Moscovium No data 116 Lv Livermorium No data 117 Ts Tennessine No data 118 Og Oganesson No data Source: Webelements
3 Exceptions to the Atomic Radius Trends
How Can You Employ This Information?
Summary: Periodic Trends Diminutive Radius
What's Next?

Well-nigh the Author
Christine graduated from Michigan State University with degrees in Environmental Biology and Geography and received her Master'southward from Duke Academy. In loftier school she scored in the 99th percentile on the Saturday and was named a National Merit Finalist. She has taught English and biology in several countries.
Source: https://blog.prepscholar.com/atomic-radius-trend
0 Response to "within a group what happens to the atomic radius"
Post a Comment